Biologically active supplements
search
news
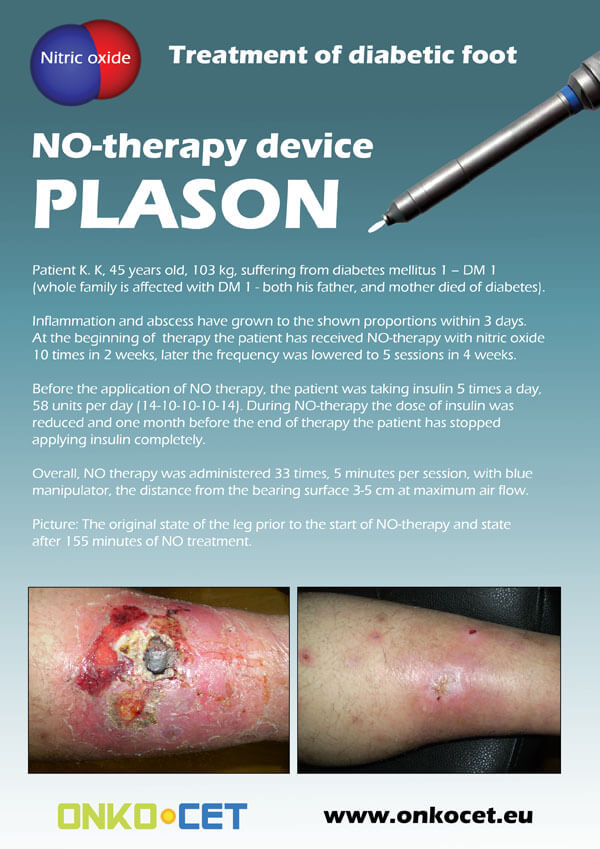
The PDF with the short report with pictures from the therapy of a diabetic foot can be viewed or downloaded here.
The pictures from the treatment of unhealing wounds an be found here:
http://www.onkocet.eu/en/produkty-detail/220/1/
The pictures from the treatment of unhealing wounds an be found here:
http://www.onkocet.eu/en/produkty-detail/293/1/
ONKOCET Ltd. has exhibited the devices from its portfolio on the MEDTEC UK exhibition in Birmingham, April 2011 through our partner Medical & Partners.

 The ONKOCET company has successfully reached the certification of yet another medical device, Infrared Camera SVIT. The Certificate can be found here. The videos from the device operation can be found here.
The ONKOCET company has successfully reached the certification of yet another medical device, Infrared Camera SVIT. The Certificate can be found here. The videos from the device operation can be found here. Our device, the non-invasive blood analyzer AMP has won the Golden Incheba prize at a medical exhibition SLOVMEDICA - NON-HANDICAP 2010. A big thank you goes to the organizers of the exhibition for acknowledging the quality of our device and to the exhibitor, the Medical & Partners company, for introduction of the AMP device to the medical public again.
Our device, the non-invasive blood analyzer AMP has won the Golden Incheba prize at a medical exhibition SLOVMEDICA - NON-HANDICAP 2010. A big thank you goes to the organizers of the exhibition for acknowledging the quality of our device and to the exhibitor, the Medical & Partners company, for introduction of the AMP device to the medical public again.We are pleased to inform our business partners, that our company has succesfully finished the certification process of Concor Soft Contact Lenses.
 You can find the certificate here.
You can find the certificate here.More information on Concor Soft Contact Lenses go to section Medical preparations/Concor soft contact lenses, or follow this link.
 Our company has finished the certification process for another medical device, computerized spirometer MAS-1K with oximeter. You can find the device certificate here.
Our company has finished the certification process for another medical device, computerized spirometer MAS-1K with oximeter. You can find the device certificate here..jpg) Since May 2010 there is a new version of AMP device available.
Since May 2010 there is a new version of AMP device available.Follow this link if you want to see the pictures and specifications of the device.
http://www.onkocet.eu/en/produkty-detail/293/1/
 Dear partners,
Dear partners, In October 2009 we have received CE certificate for another device from our portfolio, NO therapeutical device PLASON. You can find more information about this revolutionary device, used for healing of unhealing wounds, diabetic foot, or for cosmetical purposes, at our webpage, section "Medical devices" -> PLASON-NO Therapy.
.gif)
Best regards
Team of ONKOCET Ltd. company
Investigation of biological and antitumor activities
THE REPORT ON INVESTIGATION OF BIOLOGICAL AND ANTITUMOR ACTIVITIES OF C7H15MDP (GLYMURID)
1. Immunomodulatory activities of muramyl dipeptide derivatives in vitro
In all functional test-systems in vitro, amphiphilic preparation C7H15MDP (Glymurid) were compared with lipophilic homologue C16H33MDP and unmodified MDP used in equimolar concentrations.
Experimental procedures and results of the research were described in details in following papers:
1. Kalyuzhin O.V., Zemlyakov A.E. & Fuchs B.B. Distinctive immunomodulating properties and interactivity with model membranes and cells of two homologous muramyl dipeptide derivatives. // Int. J. Immunopharmacol. - 1996. - Vol. 18. - No. 11. - P. 651-659.
2. Kalyuzhin O.V., Fuchs B.B., Bovin N.V., Zemlyakov A.E. & Chirva V.Ya. Immunomodulating activities of novel muramyl dipeptide derivatives in vitro. // Bull. Exp. Biol. Med. - 1994. – Vol. 117. - No. 5. - P. 510-513.
Table 1. The influence of MDP and its derivatives on proliferation of lymphocytes in a mixed lymphocyte culture (MLC)
| Concentrations of MDP and its derivatives | |||
| 20 µ M | 10 µ M | 5 µ M | |
| Control | 11.9±0.9* | ||
| MDP | 14.6±1.0 | 14.1±0.8 | 12.7±11.2 |
| C7H15MDP | 15.9±1.4 | 16.4±10.6 | 14.5±0.9 |
| C16H33MDP | 5.9±0.8 | 7.7±0.4 | 9.5±1.2 |
*Each value in the table is the mean index of proliferation stimulation of lymphocytes in MLC + S.D. of six independent experiments.
Table 2. LPS-induced proliferation of mouse splenocytes under the influence of MDP and its derivatives
| Concentrations of MDP and its derivatives | |||
| 20 µ M | 10 µ M | 5 µ M | |
| Control | 3.4±0.2* | ||
| MDP | 4.1±0.5 | 4.4±0.1 | 4.1±0.4 |
| C7H15MDP | 4.4±0.3 | 4.6±0.3 | 4.4±0.3 |
| C16H33MDP | 3.0±1.0 | 2.6±0.3 | 3.0±0.1 |
*Each value in the table is the index of proliferation of lymphocytes in response to stimulation of LPS (1 mg/ml) under the influence of MDP derivatives (mean ± S.D. of three independent experiments).
Table 3. The influence of MDP and its derivatives on the generation of allospecific cytotoxic T lymphocytes (CTLs) in a mixed lymphocyte culture
| Concentrations of MDP and its analogues (µM) | Effector:target cells | |||
| 30:1 | 10:1 | 3:1 | ||
| Control | 71±5 | 51±3 | 22±4 | |
| 20 | MDP | 82±3 | 60±2 | 31±3 |
| C7H15MDP | 94±4 | 73±4 | 46±6 | |
| C16H33MDP | 35±17 | 23±6 | 9±3 | |
| 10 | MDP | 81±2 | 64±5 | 30±6 |
| C7H15MDP | 87±6 | 74±4 | 30±6 | |
| C16H33MDP | 52±13 | 27±6 | 11±9 | |
| 5 | MDP | 77±7 | 58±7 | 27±3 |
| C7H15MDP | 83±5 | 62±4 | 35±7 | |
| C16H33MDP | 69±10 | 42±7 | 19±1 | |
Each value in the table is the cytotoxic activity of CTLs (%) with respect to allogenic tumor cells P815 (mean ± S.D. of three independent experiments)
Table 4. Production of IL-1 (a) and TNF (b) by C57BL/6 mouse peritoneal macrophages cultured in presence of MDP and its derivatives
| Concentrations of MDP and its derivatives | |||
| 20 µ M | 10 µ M | 5 µ M | |
| (a) Proliferation of tymocytes cultured with samples of IL-1 (index of proliferation) | |||
| Control | 1.3±0.4 | ||
| MDP | 3.9±0.3 | 3.8±0.3 | 3.4±0.2 |
| C7H15MDP | 4.8±0.2 | 3.9±0.2 | 3.8±0.2 |
| C16H33MDP | 3.9±0.1 | 3.9±0.4 | 3.8±0.1 |
| (b) Cytosis of L929 cells by samples of TNF (%) | |||
| Control | 7±6 | ||
| MDP | 45±4 | 45±3 | 40±3 |
| C7H15MDP | 62±5 | 57±8 | 41±2 |
| C16H33MDP | 47±10 | 40±16 | 36±15 |
Each value in the table is the mean ± S.D. of three independent experiments
Table 5. The influence of MDP and its derivatives (10 ?M) on cytotoxic activity of natural killers (NK) with respect to NK-sensitive lymphoma YAC-1 cells
| MDP and its derivatives | Cytotoxicity (%) |
| Control | 24±2 |
| MDP | 28±2 |
| C7H15MDP | 31±2 |
| C16H33MDP | 21±3 |
Each value in the table is mean index of cytotoxicity ± S.D. of three independent experiments.
Conclusion
Amphiphilic preparation ?-heptylglycoside-MDP (C7H15MDP, Glymurid) has been shown in model test systems in vitro to have a more significant stimulatory effect on the main populations of immunocompetent cells including cells-effectors of antitumor response compared to unmodified MDP and lipophilic analogue (?-hexadecylglycoside-MDP). C7H15MDP has been found to stimulate lymphocyte proliferation in a mixed lymphocyte culture (Table 1), to increase cytotoxicity of natural killers (NK) (Table 5), to activate allospecific cytotoxic T-lymphocytes (CTTL) generation (Table 3) and interleukin-1 (IL-1) and tumor necrosis factor (TNF) production by macrophages (Table 4) and to possess a comitogenic activity with B cell mitogen lipopolisaccharide (Table 2).
2. The interaction of the MDP derivatives with model membranes and their uptake by cells
Experimental procedures and results of the research were described in details in following papers:
1. Kalyuzhin O.V., Zemlyakov A.E. & Fuchs B.B. Distinctive immunomodulating properties and interactivity with model membranes and cells of two homologous muramyl dipeptide derivatives. // Int. J. Immunopharmacol. - 1996. - Vol. 18. - No. 11. - P. 651-659.
2. Kalyuzhin O.V. & Fuchs B.B. The influence of hydro-lipophilic balance of muramyl dipeptide derivatives on their interactivity with biological membranes and uptake by cells. // Bull. Exp. Biol. Med. - 1996. - Vol. 122. - No. 12. - P. 658-661.
In order to explain distinctions in immunomodulatory activities of amphiphilic and lipophilic homologues, the interaction of these muramylpeptides with several model membranes has been studied.
Hydro-lipophilic balance of the preparations can significantly influence the interaction of homologous MDP derivatives with cell membranes and their internalization into cells as well as intracellular traffic. To demonstrate the differences in hydro-lipophilic balance of two homologues, the simplest model water/chloroform "membrane" has been used.
Marked differences in distribution of 14C-labelled preparations in the phases of this model "membrane" have been found. 64% of C7H15MDP was included in the water phase, and 36% of the preparation in the organic phase. At the same time, the water phase included only 28% of its lipophilic homologue C16H33MDP, and the organic phase 72%.
The other model lipid membrane prepared by means of dipping of filter discs in equimolar mixture of cholesterol and phosphatidylcholine in bensol has been used to study kinetics of the MDP derivatives from one water phase to the other and delay of the preparations in lipid barrier.
Preparation C16H33MDP appeared in trace quantity in the second water phase in 1 h from the beginning of the experiment, and in 6 h its concentration in this phase became significant. But its further penetration through lipid barrier was significantly slower in comparison with C7H15MDP. The former appeared in the second water phases only in 24 h. However, speed of its penetration then increased and after 72 h of the experiment C7H15MDP was thus the first to reach a state of equilibrium between the preparation concentrations in water phases from both sides of the model membrane.
Detection of membrane-associated radioactivity after the end of the experiment revealed that C7H15MDP bound to the model membrane four times less intensively than its lipophilic analogue.
To understand better how the immunomodulators-derivatives of MDP interact with biological membranes and penetrate into cells, the uptake of the 14?-labelled immunomodulators (C7H15MDP and C16H33MDP) by human erythroleukemia K562 cells have been investigated.
Although the uptake of immunomodulators by cells depends not only on the physicochemical properties of the preparation but also on some other factors, the results of this set of experiments correlated in general with the data on the interaction of the MDP derivatives with the model cholesterol/phospholipid membrane.
One hour after the start of cell cultivation in a medium lacking glutamine (for suppression of cell division) and containing radiolabelled MDP analogues, the lipophilic derivative C16H33MDP bound to the cells 50% more intensively than its amphiphilic homologue (p<0.05). However, C16H33MDP uptake then slowed down and after 2 h of incubation the cell-associated radioactivity practically entered the "plateau" stage. C7H15MDP, which bound to the cells not so intensively at the beginning of the experiment, did not enter the phase of equilibrium between the uptake and efflux after 2-4 h. As a result, in 24 h the uptake of C7H15MDP by cells exceeded by 25% the uptake of its more lipophilic analogue (p<0.05).
Thus, significant quantity of C16H33MDP delayed in the plasma membrane. Of course, lipophilic preparation associated with plasma membrane is to be internalized actively by cells as a result of endocytosis. However, the exocytosis evidently soon balances this process.
On the contrary, amphiphilic C7H15MDP, which was included relatively slowly into the membranes at the beginning, then left easily, which finally gave it advantages in penetration through lipid membrane and in the uptake by cells, compared to the more lipophilic C16H33MDP. Amphiphilicity of the preparation C7H15MDP is a principal character that ensures an effective penetration through biological membranes, which correlates with marked activation of the main cells-effectors of antitumor immune response.
3. Antitumor activities of C7H15MDP in mouse lymphoma and melanoma models
Experimental procedures
Briefly, suspension of lymphoma EL-4 or melanoma B16 cells (106 cells) was injected subcutaneously into the footpad of C57BL/6 mice. Then mice underwent course of C7H15MDP treatment. Solution of the preparation in phosphate buffer saline (PBS) was administered i.v. or p.o. Single dosage for p.o. administration was of 1 mg/kg, and of 0.5 mg/kg when administered i.v. The preparation was administered twice a week during 3 weeks, beginning immediately after implantation of tumor cells.
Control mice were treated with PBS without active compound.
Antitumor effect was estimated using the following criteria: (1) prolongation of life-span, (2) inhibition of tumor growth, which was evaluated by detection of tumor size on day 21 of the experiment.
Table 6. The influence of C7H15MDP on life-span of tumor-bearing mice
| Tumor | Treatment | Mean life-span (days) | Prolongation of life-span (%) |
| (a) Melanoma ? 16 | |||
| Control (PBS p.o.) | 30±4 | - | |
| Control (PBS i.v.) | 36±3 | - | |
| C7H15MDP p.o | 51±5 | 70 | |
| C7H15MDP i.v. | 74±9 | 101 | |
| (b) Lymphoma EL-4 | |||
| Control (PBS p.o.) | 42±6 | - | |
| Control (PBS i.v.) | 39±4 | - | |
| C7H15MDP p.o | 50±7 | 19 | |
| C7H15MDP i.v. | 63±5 | 62 | |
Each value in the table is the mean ± S.D. of three independent experiments.
Table 7. Inhibition of tumor growth under the influence of C7H15MDP
| Tumor | Treatment | Tumor node volume at the day 21 after tumor inoculation (mm3) | Inhibition of tumor growth (%)
|
| (a) Melanoma B 16 | |||
| Control (PBS p.o.) | 765±69 | - | |
| Control (PBS i.v.) | 736±51 | - | |
| C7H15MDP p.o. | 571±65 | 25 | |
| C7H15MDP i.v. | 494±49 | 33 | |
| (b) Lymphoma EL-4 | |||
| Control (PBS p.o.) | 842±78 | - | |
| Control (PBS i.v.) | 789±64 | - | |
| C7H15MDP p.o. | 650±73 | 23 | |
| C7H15MDP i.v. | 463±52 | 41 | |
Each value in the table is the mean ± S.D. of three independent experiments.
Conclusion
Thus, the preparation has significant antitumor activities, which have been demonstrated by prolongation of life-span of tumor-bearing animals (Table 6) and inhibition of tumor growth (Table 7). Further trials of the preparation have shown that the treatment, when begun 1 week before tumor implantation, was more successful, and complete regression of tumor was observed in several cases (data not shown). On the contrary, the delay of treatment beginning caused a decrease of antitumor efficacy. Also we have demonstrated marked antimetastatic activities of Glymurid (data not shown).
Investigation of biological and antitumor activities of Glymurid (C7H15MDP) points to the expediency of its clinical trials as immunomodulator, particularly as stimulator of antitumor immune response.