Medical devices
search
news
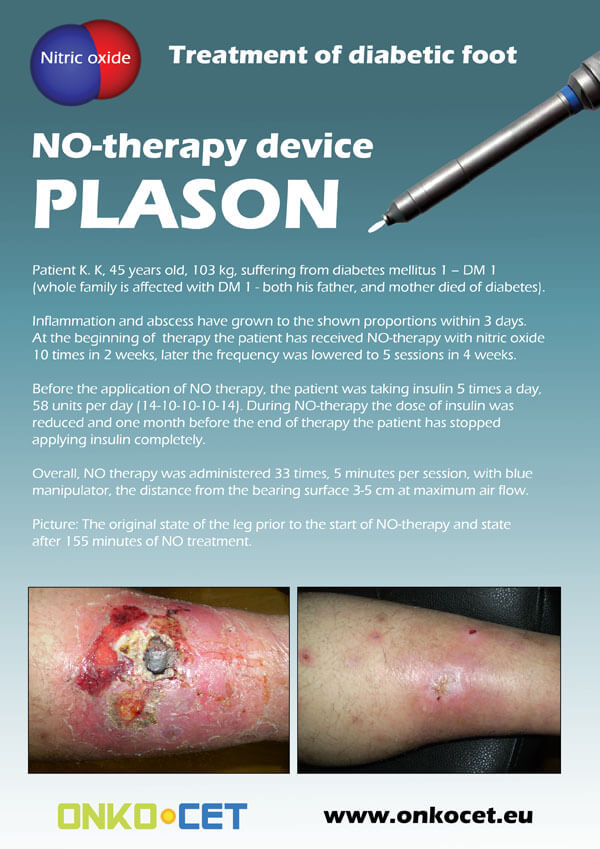
The PDF with the short report with pictures from the therapy of a diabetic foot can be viewed or downloaded here.
The pictures from the treatment of unhealing wounds an be found here:
http://www.onkocet.eu/en/produkty-detail/220/1/
The pictures from the treatment of unhealing wounds an be found here:
http://www.onkocet.eu/en/produkty-detail/293/1/
ONKOCET Ltd. has exhibited the devices from its portfolio on the MEDTEC UK exhibition in Birmingham, April 2011 through our partner Medical & Partners.

 The ONKOCET company has successfully reached the certification of yet another medical device, Infrared Camera SVIT. The Certificate can be found here. The videos from the device operation can be found here.
The ONKOCET company has successfully reached the certification of yet another medical device, Infrared Camera SVIT. The Certificate can be found here. The videos from the device operation can be found here. Our device, the non-invasive blood analyzer AMP has won the Golden Incheba prize at a medical exhibition SLOVMEDICA - NON-HANDICAP 2010. A big thank you goes to the organizers of the exhibition for acknowledging the quality of our device and to the exhibitor, the Medical & Partners company, for introduction of the AMP device to the medical public again.
Our device, the non-invasive blood analyzer AMP has won the Golden Incheba prize at a medical exhibition SLOVMEDICA - NON-HANDICAP 2010. A big thank you goes to the organizers of the exhibition for acknowledging the quality of our device and to the exhibitor, the Medical & Partners company, for introduction of the AMP device to the medical public again.We are pleased to inform our business partners, that our company has succesfully finished the certification process of Concor Soft Contact Lenses.
 You can find the certificate here.
You can find the certificate here.More information on Concor Soft Contact Lenses go to section Medical preparations/Concor soft contact lenses, or follow this link.
 Our company has finished the certification process for another medical device, computerized spirometer MAS-1K with oximeter. You can find the device certificate here.
Our company has finished the certification process for another medical device, computerized spirometer MAS-1K with oximeter. You can find the device certificate here..jpg) Since May 2010 there is a new version of AMP device available.
Since May 2010 there is a new version of AMP device available.Follow this link if you want to see the pictures and specifications of the device.
http://www.onkocet.eu/en/produkty-detail/293/1/
 Dear partners,
Dear partners, In October 2009 we have received CE certificate for another device from our portfolio, NO therapeutical device PLASON. You can find more information about this revolutionary device, used for healing of unhealing wounds, diabetic foot, or for cosmetical purposes, at our webpage, section "Medical devices" -> PLASON-NO Therapy.
.gif)
Best regards
Team of ONKOCET Ltd. company
Report on Medical Testing

Report on Medical Testing
Clinical Trials ANESA 2013
REPORT ON CLINICAL STUDIES OF THE MEDICAL DEVICE
“Automatic Noninvasive Express Screening Analyzer ANESA”
Kharkiv, 21-05-2013
Materials on clinical study of the medical device “Automatic Noninvasive Express Screening Analyzer ANESA”, manufactured by “Biopromin” Ltd. (Ukraine) are provided. It is designed to collect data of a patient's clinical blood analysis values (hemoglobin, glucose, aspartate aminotransferase (AST), alanine aminotransferase (ALT), amylase, total protein, total bilirubin, direct bilirubin, ceruloplasmin, hematocrit, creatinine, leucocytes, lymphocyte, urea, monocytes, erythrocyte sedimentation rate (ESR), differential count – band neutrophils) without invasive blood drawing followed by data processing with “USPIH 10” software.
Clinical study of volunteers was carried out by comparative analysis with using certified hematology analyzers and commonly accepted methods of laboratory examinations. Study results were processed by statistical methods.
This study allowed determining that the test medical device ensures the necessary reproducibility with 95% of confidence interval. Parallel control by the standard (common) laboratory methods is recommended.
More information available in:
ANESA Clinical trial 2013.pdf
The producer of ANESA device held several clinical trials in various countries - Ukraine, Russia, Hungary (EU), Belarus, China, etc.
All the clinics and laboratories were assigned by the respective Notified Body.
Some of the trials were done after registration of the device; they must be characterized as post-clinical trials.

CLINICAL TRIALS ANESA.pdf
PDF File: 150 KB
Biopromin_Medical_Test_Program_En.pdf
PDF File: 140 KB
Biopromin_Medical_Test_Report_En.pdf
PDF File: 150 KB
Biopromin_Medical_Test_Report_Tables_En.pdf
PDF File: 85 KB
Medical Trial Report Moscow Oncological Research Institute En.pdf
PDF File: 400 KB
Clinical Evaluation Hungary.pdf
PDF File: 2,3 MB