Medical devices
search
news
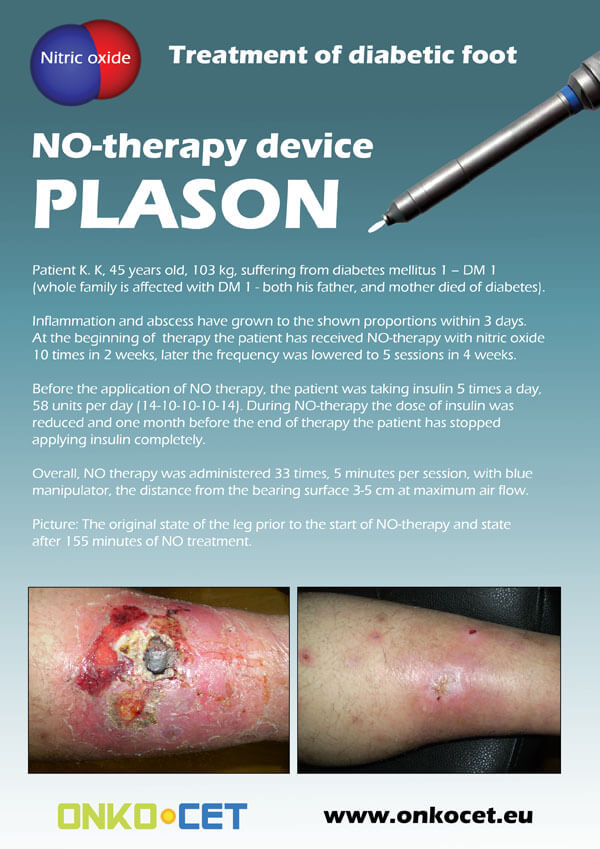
The PDF with the short report with pictures from the therapy of a diabetic foot can be viewed or downloaded here.
The pictures from the treatment of unhealing wounds an be found here:
http://www.onkocet.eu/en/produkty-detail/220/1/
The pictures from the treatment of unhealing wounds an be found here:
http://www.onkocet.eu/en/produkty-detail/293/1/
ONKOCET Ltd. has exhibited the devices from its portfolio on the MEDTEC UK exhibition in Birmingham, April 2011 through our partner Medical & Partners.

 The ONKOCET company has successfully reached the certification of yet another medical device, Infrared Camera SVIT. The Certificate can be found here. The videos from the device operation can be found here.
The ONKOCET company has successfully reached the certification of yet another medical device, Infrared Camera SVIT. The Certificate can be found here. The videos from the device operation can be found here. Our device, the non-invasive blood analyzer AMP has won the Golden Incheba prize at a medical exhibition SLOVMEDICA - NON-HANDICAP 2010. A big thank you goes to the organizers of the exhibition for acknowledging the quality of our device and to the exhibitor, the Medical & Partners company, for introduction of the AMP device to the medical public again.
Our device, the non-invasive blood analyzer AMP has won the Golden Incheba prize at a medical exhibition SLOVMEDICA - NON-HANDICAP 2010. A big thank you goes to the organizers of the exhibition for acknowledging the quality of our device and to the exhibitor, the Medical & Partners company, for introduction of the AMP device to the medical public again.We are pleased to inform our business partners, that our company has succesfully finished the certification process of Concor Soft Contact Lenses.
 You can find the certificate here.
You can find the certificate here.More information on Concor Soft Contact Lenses go to section Medical preparations/Concor soft contact lenses, or follow this link.
 Our company has finished the certification process for another medical device, computerized spirometer MAS-1K with oximeter. You can find the device certificate here.
Our company has finished the certification process for another medical device, computerized spirometer MAS-1K with oximeter. You can find the device certificate here..jpg) Since May 2010 there is a new version of AMP device available.
Since May 2010 there is a new version of AMP device available.Follow this link if you want to see the pictures and specifications of the device.
http://www.onkocet.eu/en/produkty-detail/293/1/
 Dear partners,
Dear partners, In October 2009 we have received CE certificate for another device from our portfolio, NO therapeutical device PLASON. You can find more information about this revolutionary device, used for healing of unhealing wounds, diabetic foot, or for cosmetical purposes, at our webpage, section "Medical devices" -> PLASON-NO Therapy.
.gif)
Best regards
Team of ONKOCET Ltd. company
Accuracy and reliability
RELIABILITY, ACCURACY AND SAFETY OF MEDICAL DEVICES
In the beginning of 2013 on the site of ResearchGate https://www.researchgate.net/ Phil Petursson raised a question: How do you know that medical devices are reliable, accurate, and safe?
Petursson wrote: “I believe that approval ratings that include: 'CE', 'UL', and 'GS' are only electrical safety ratings, and do not evaluate the reliability and validity (accuracy) of a medical device.“
Is it true that the CE mark on medical devices (MD) means only electrical safety ratings?
Before any producer of medical device labels his product with the CE mark and introduces them on EU market with medical devices, the device must fulfill the conditions written in Directive 93/42/EEC (MDD), 98/79/EC (IVDD) or 90/385/EEC (AIMDD). In practice this process is called „CE registration“.
What does the process of CE registration mean?
The process of CE registration is divided into two parts. First, each producer or EU representant of a manufacturer is obliged to prepare a so called Technical Construction File (TCF).
PDF File: Reliability of medical devices.pdf
Size: 3.4 MB