Medical devices
search
news
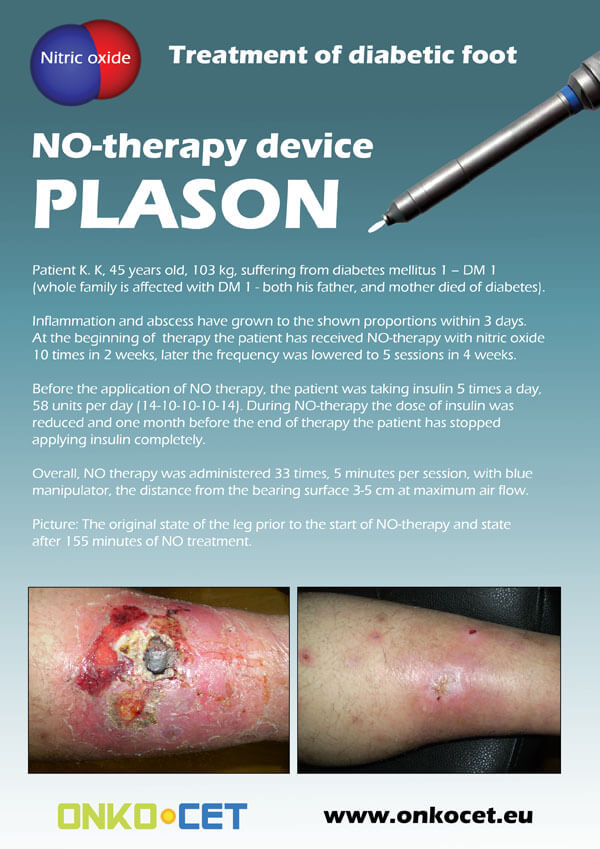
The PDF with the short report with pictures from the therapy of a diabetic foot can be viewed or downloaded here.
The pictures from the treatment of unhealing wounds an be found here:
http://www.onkocet.eu/en/produkty-detail/220/1/
The pictures from the treatment of unhealing wounds an be found here:
http://www.onkocet.eu/en/produkty-detail/293/1/
ONKOCET Ltd. has exhibited the devices from its portfolio on the MEDTEC UK exhibition in Birmingham, April 2011 through our partner Medical & Partners.

 The ONKOCET company has successfully reached the certification of yet another medical device, Infrared Camera SVIT. The Certificate can be found here. The videos from the device operation can be found here.
The ONKOCET company has successfully reached the certification of yet another medical device, Infrared Camera SVIT. The Certificate can be found here. The videos from the device operation can be found here. Our device, the non-invasive blood analyzer AMP has won the Golden Incheba prize at a medical exhibition SLOVMEDICA - NON-HANDICAP 2010. A big thank you goes to the organizers of the exhibition for acknowledging the quality of our device and to the exhibitor, the Medical & Partners company, for introduction of the AMP device to the medical public again.
Our device, the non-invasive blood analyzer AMP has won the Golden Incheba prize at a medical exhibition SLOVMEDICA - NON-HANDICAP 2010. A big thank you goes to the organizers of the exhibition for acknowledging the quality of our device and to the exhibitor, the Medical & Partners company, for introduction of the AMP device to the medical public again.We are pleased to inform our business partners, that our company has succesfully finished the certification process of Concor Soft Contact Lenses.
 You can find the certificate here.
You can find the certificate here.More information on Concor Soft Contact Lenses go to section Medical preparations/Concor soft contact lenses, or follow this link.
 Our company has finished the certification process for another medical device, computerized spirometer MAS-1K with oximeter. You can find the device certificate here.
Our company has finished the certification process for another medical device, computerized spirometer MAS-1K with oximeter. You can find the device certificate here..jpg) Since May 2010 there is a new version of AMP device available.
Since May 2010 there is a new version of AMP device available.Follow this link if you want to see the pictures and specifications of the device.
http://www.onkocet.eu/en/produkty-detail/293/1/
 Dear partners,
Dear partners, In October 2009 we have received CE certificate for another device from our portfolio, NO therapeutical device PLASON. You can find more information about this revolutionary device, used for healing of unhealing wounds, diabetic foot, or for cosmetical purposes, at our webpage, section "Medical devices" -> PLASON-NO Therapy.
.gif)
Best regards
Team of ONKOCET Ltd. company
Technical Requirements for MEIK usage
TECHNICAL REQUIREMENTS FOR USAGE OF ELETRICAL IMPEDANCE MAMMOGRAPH "MEIK®" FOR BREAST CANCER DIAGNOSIS
Product: Electroimpedance Computer Mammograph “MEIK”®
Model/type: version 5.0
Classification: medical device class IIa
The electrical impedance computer mammograph "MEIK®".
The mammograph makes it possible to visualize distribution of biological tissues electrical conductivity in several lateral sections of patient's body and to detect on the images tumors as regions with abnormal values of electrical conductivity. The main advantages of electrical impedance methods of diagnostics are absolute safety of examination, comprehensiveness, connected with significant level of correlation of electrical conductivity (electrical impedance) of biological tissues and their physiological state, compactness, reasonable price and simplicity of examination procedure.
Benefits
The electrical impedance diagnosis can be performed for patients of all sex (for women from the first ovulation period up to death), ages and can be used for screening of breast cancer diseases. Therefore the doctor can perform repeated examinations, monitor the disease dynamics as well as effects of changes of tissue conductivity during drug therapy.
Side Effects
During the scanning process the device, utilizing one out of 256 electrodes of the matrix successively injects into the patient's body weak alternative electric current (0,5 mA) with the frequency of 50 kHz and registers corresponding distributions of electric potentials on its surfaces. It is absolutely harmless for the patients as well as for the medical staff. By usage of the electro impedance mammograph MEIK there are no side effects.
Patents for the invention
The electrical impedance mammograph "MEIK®" is protected by the Eurasian patent of the for the invention # 012006.
Approval documentations
The product, an electrical impedance computer mammograph "MEIK®", is manufactured according to the Technical Conditions TU 9442-001-21702999-2013.
On the decision of the "Committee for Equipment and Technical Instrumentation, Utilized in Oncology and Medical Radiology" the device has been recommended for batch production and application in medical practice. It is registered in the State Register of Medical Goods. (registration certificate # 29/05010303/5420-03 dated July 2003), OKP 94 4220, class 2 b.
The electrical impedance mammograph "MEIK®" possesses the Conformance Certificate # ROSS RU.IM02.?14247 of the Government Standard of Russian and CE1023 ?07 0681 QS/NB.
The electrical impedance mammograph "MEIK®" corresponds to sanitary norms, that are confirmed by sanitary-and-epidemiologic conclusion #77.99.34.944.D.007393.08.06.
Requirement for technician
The electrical impedance computer mammograph "MEIK®" is intended for noninvasive measuring conductivity in biological tissues especially breast tissue.
It is utilized in oncology; the monitoring of treatment as well as drugs and physiotherapy procedure effects. Doctor should be specialized in radiology, oncology, gynecology, immunology and surgery, with training in electrical impedance in biological tissues (MEIK certificate).
Conclusion
Medical device mammograph MEIK can be used by the doctor specialists graduated through training in using electrical impedance mammograph and received certificate for the training published by producer company SIM-technika Ltd., Yaroslavl, Russia or by its representative on territory of the European Union company ONKOCET Ltd., Bratislava, Slovakia, www.onkocet.eu .
The device specifications are the following:
The electrical impedance computer mammograph "MEIK®" is recommended for using in medicine practice for oncology screening; at specialized oncology and mammology centers for detection of cancer and the monitoring of treatment.
Storage
For short term of storage the radiometer must be stored indoors at 10-35°C temperature, and relative humidity must be less 70% at room temperature of 25°C. Long term storage of the radiometer should be provided in warehouse of a manufacturer or customer in accordance with storage conditions of the national standards GOST 15.150.
Service & Warranty
The manufacturer guarantees that the radiometer corresponds to indicated specifications when all conditions of use, technical service, transporting and storing requirements indicated in the device documents are met.
The warranty period is 24 months, it begins once the radiometer is set to be used.